|
Frequently the artist had conceived of the patterns or arrangements before
the scientists had found their counterparts in the infra- or ultra-visible
realms. The conceptual capability of the artists' intuitive formulation
of the evolving new by subconscious coordinations are tremendously important.
(Fuller, "Utopia or Oblivion," 111) |
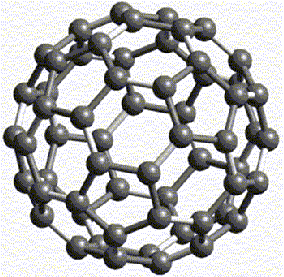
Figure 16: Buckyball
In the 1960s, Gyorgy Kepes, then Director of the Centre for Visual Studies
at MIT, took uniformly sized black and white photographs of non-representational
paintings by many artists. He mixed them all together with the same size black
and white photographs taken by scientists of all kinds of phenomena through
microscopes and telescopes. Then, together with his students, he classified
the mixed up photographs by pattern types. What they found is not only that
it was difficult to distinguish which was art and which was science, but when
they looked at the backs of many art pieces, frequently they predated the scientific
counterpart (Fuller, "Utopia or Oblivion," 113).
Buckminster Fuller related this story in one of his lectures and had a similar
experience in 1962 when chemist Sir Aaron Klug observed geodesic structuring
of viruses and wrote to Fuller telling him of his discovery. Fuller wrote back
immediately with the formula for the number of nodes on a shell (10f + 2, varying
according to frequency) as confirmation of Klug's hypothesis, and Klug answered
that the values were consistent with the virus research (Edmonson 239). It is
important to note that geodesic domes were utilised worldwide fifteen years
before electron microscopy enabled detection of virus capsids. In 1982, Klug
won a Nobel Prize for his "structural elucidation of important nucleic
acid-protein complexes," and has been described as a "biological map
maker," a Magellan "charting the infinitely complex structures of
body's largest molecules" ("A Map Maker of Molecules").
A much more dramatic proof of Fuller's inventions was demonstrated only a year
after his death when a carbon molecule remarkably resembling his structures
was discovered by a group of scientists working at Rice University in Houston,
Texas. During an experiment involving the use of laser beams to evaporate graphite,
scientists Harry Kroto, Rick Smalley, Bob Cur and their students, identified
a set of conditions in which the C60 species could be produced in an incredible
numbers relative to any other cluster. The extraordinary stability of the molecule
prompted the researchers to look its structure. When the researchers recalled
the structure of the US Pavilion at the Montreal Expo '67, it helped them realize
that the molecule consists of twelve regular, same-size pentagons and twenty
regular, same-size hexagons (Kroto 162-163). This molecule is the third modification
of carbon to be discovered, the other two being graphite and diamond.
What makes this discovery unique is that it occurred from the merging of two
separate lines of research. Kroto was investigating the composition of mysterious
long chain carbon molecules that have been detected in stardust, and was particularly
interested in how such molecules might form in the outer flares of stars. He
learned that Smalley had built a laser-powered device that would vaporise almost
any substance and travelled to Rice to use it. The graphite experiment combined
their experiences and interests and brought cluster physics and astrophysics
together in a chemical exercise. The group discovered that when they vaporised
carbon in a chamber filled with inert helium gas, an extremely strange thing
happened: the carbon molecules formed into clusters, most of which contained
60 atoms, and they were so stable that the scientists could only theorize that
the molecules must have arranged themselves into hollow, closed shells. When
the scientists reported their discovery of the globular framework, the findings
met with considerable scepticism. At the time, most chemists would have said
that such a structure could not exist as a stable molecule. It was assumed that
any such configuration would have to be flat (Supple A3). But by 1990, other
labs had begun making the clusters in bulk, and the ball shape was confirmed.
In addition, numerous forms were found, composed of interlocking hexagons and
pentagons. The number of variations may be infinite. The hollow shape of the
molecules provides a convenient container for one or more atoms of other elements,
thus allowing for many new substances to emerge.
In honour of Fuller, these molecular clusters were named "buckminsterfullerenes"
by Kroto and Smalley, and were later nicknamed "buckyballs." Their
discovery spurred a revolution in carbon chemistry and a profusion of new materials:
polymers, catalysts, and drug-delivery systems. The discovery has also been
important to research in physics and has resulted in novel insights into superconducting
substances and may also help explain the origin of the cosmos (Zimmer 30). In
1996, Robert F. Curl, Jr., Richard F. Smalley from Rice University in Houston,
and Harold W. Kroto from the University of Sussex in England, shared a Nobel
Prize for their collaborative discovery (Supple A3).
Although the discoverers were honoured with a Nobel Prize, there are a few
who have pointed to this possibility earlier, but received no response. In the
same way that Fuller's message of stable structures that utilize the tensegrity
principles was too early for its time, the discovery of the molecule had to
wait. As early as 1966, David Jones of the UK considered the possibility of
graphite sheets curling up into hollow ball-like molecules. In 1970, Eiji Osawa
of Japan suggested the existence of C60 with a truncated icosahedral shape based
purely on symmetry considerations. In 1973, D.A. Bochvar and Elena G. Galpern
of Moscow carried out some theoretical calculations that led them to postulate
the great relative stability of a 60 molecule with a truncated icosahedral shape
(Hargittai 336).
Whereas cells were regarded as the basic building blocks of living organisms
during the nineteenth century, the attention shifted from cells to molecules
toward the middle of the twentieth century when geneticists began to explore
the molecular structure of the gene. Biologists were discovering that the characteristics
of all living organisms-from bacteria to humans-were encoded in their chromosomes
in the same chemical substance and using the same code script. After two decades
of research, biologists have unravelled the precise details of this code. But
while they may know the precise structure of a few genes, they know very little
of the ways these genes communicate and cooperate in the development of an organism.
Similarly, computer scientists may be well versed in networked technologies
but have no clue as to how and why the Internet exploded as it did-organically
and spontaneously.